 |
|||
|
|
|
How to Mix Photographic Developers
THEORY Oxides are binary compounds formed with oxygen and other elements. Hydrides are binary compounds formed with hydrogen and other elements. Acids are loosely defined as oxides (sometimes hydrides) of non-metallic elements dissolved in water. Acids are typically corrosive, sour to the taste, turn litmus paper red, react with some metals to liberate hydrogen, and neutralize bases. A broader definition of an acid is any substance which ionizes in water to yield positive hydrogen ions; or any compound which can transfer a proton to another compound. The halogens (fluorine, chlorine, bromine, and iodine) can combine directly with hydrogen to form the strong acids, hydrofluoric, hydrochloric, hydrobromic, and hydriodic; the latter three are particularly important in the manufacture of photographic emulsions. Bases (or alkaline compounds) are the opposite of acids and are loosely defined as oxides of metallic elements dissolved in water. They are typically corrosive, slippery like soap, and change litmus paper blue. A broader definition of a base is any substance which ionizes in water to yield negative hydroxide ions; or any compound which can accept a proton from another compound. Salts are chemical compounds formed when acids are combined with bases, a process known as neutralization.
Inorganic Compounds typically contain metallic elements that vaporize at high temperatures leaving an ash residue. Sulfuric, nitric, hydrochloric, perchloric, hydrofluoric, hydrobromic, phosphoric, sulfurous, hypochlorous, and arsenic acids are all inorganic, as are the hydroxides of sodium, potassium, calcium, copper, iron, and other metals. Organic Compounds are compounds with carbon that typically contain other non-metallic elements (particularly hydrogen, oxygen, nitrogen, sulfur, and sometimes phosphorus) that burn, most often producing a carbon residue. Many organic compounds are used in photographic processing, notably as developing agents. Organic acids include acetic, butyric, benzoic, oleic, carbolic, ascorbic, among others. Organic bases include methylamine, aniline, and pyridine. Amphoteric Compounds have both slightly acidic and slightly basic properties. Chief among these for photographic purposes is water, but also included are zinc and aluminum oxides. The Photographic Emulsion
Black and white photographic emulsions are composed of layers of silver halide crystals, with additional small amounts of various sensitizing agents, suspended in gelatin. Exposure and Development The silver halide crystals in a photographic emulsion are not perfect. There are dislocations in the crystalline structure which contain free silver ions, known as sensitivity centers. Light, striking the silver halide crystals, raises the energy level of the halide molecules, allowing electrons to move freely through the crystals. These electrons are drawn to the positively charged silver ions and combine with them to form atoms of pure silver. When this happens, the sensitivity centers are referred to as latent image specks. During development there are actually several chemical reactions taking place alongside each other in a very complex manner. The developing agent is always dissolved in an alkaline solution to form its sodium salt, which ionizes in water. In the presence of this ionized developing agent, a cascade effect is induced around the sensitivity specks, wherein nearby molecules of silver halide are ionized and split into their components, allowing ever more silver ions to combine with electrons, and liberating bromide, chloride, and iodide. Eventually all of the exposed silver halide crystal is reduced to pure silver. After reduction, the units of silver are referred to as grains. Sometimes the development reaction becomes so vigorous that it carries over to unexposed silver halide crystals. This is known as contagious development, producing what is commonly referred to as fog. For further information on photographic emulsions and the development process, see The Photographic Latent Image on the Kodak website. Components of Photographic Developers Developing agents are organic chemical reducers with an affinity for oxygen which can liberate
metals from their salts--they convert the silver halides in photographic emulsions to pure silver, as described above. Most modern developing agents (with the exception of ascorbic acid and
phenidone) consist of complex organic hydrocarbons of the benzene series, referred to as aromatic hydrocarbons, most of which are extracted from coal-tar.
Factors to be considered when evaluating the various developing agents include solubility in water , tendency to cause fog, staining effect, sensitivity to temperature changes, reaction to bromides, sensitivity to the pH of the solution, keeping properties, effect on grain size, and toxicity.
Phenidone (1-phenyl-3-pyrazolidone), its potential as a developer discovered by J. D. Kendall in 1940, is a clean-working,
Amidol (2,4-Diaminophenol hydrochloride), introduced in 1892, is a low-fog agent which
dissolves easily in water and sulfite solutions, and is moderately sensitive to bromides. A powerful, rapid developing agent, amidol is toxic, stains the fingers with oxidation by-products,
and only keeps for a couple of days in solution, though its keeping properties Pyrocatechin (o-Dihydroxybenzene), also known as Catechol or Catechin, is a toxic, staining, low
-fog agent that dissolves easily in warm water and is only slightly sensitive to bromide in its most common solution with Pyrogallol (1,2,3-Trihydroxybenzene) was introduced in 1851 and is commonly referred to as
pyro or (incorrectly) pyrogallic acid. Pyrogallol is a staining agent that is quite toxic, produces low fog, and is moderately sensitive to the addition of bromide. Used by itself it is very soft working, but
p-Phenylenediamine (4-diaminobenzene) was introduced in 1888, but was not used exentsively until the There are other known developing agents, including Chlorquinol (Chlorhydro-quinone) or Adurol; orthoaminophenol; Ortol which is a combination of hydroquinone and the sulfate of methyl -orthoaminophenol; gallacetophenenone, ferric oxalate, etc. These agents are not often used in the home darkroom, though they may find commercial applications. There is also a chemical of the naphthalene series (a double benzene ring) known as Eikonogen (1-amino-2-naphthol-6-sulphonic acid) which is obsolete as a developing agent. Preservatives prevent undue oxidation of the developing agent. Sodium sulfite is most widely used , though it has a pronounced solvent action which tends to increase fog, so that occasionally sodium bisulfite*, sodium metabisulfite, or potassium metabisulfite are called for. Generally, the amount of sulfite used in developers is kept to the minimum necessary to retard oxidation, unless solvent action is desired for a "fine-grain" effect. The metabisulfites are said to eliminate the solvent action, as they are distinctly acidic. Ascorbic acid may also be used as a preservative.
Restrainers both slow the rate of development and prevent unwanted fog. Potassium bromide is
almost universally used, and has the additional effect of warming print color when used in paper developers. Sodium bromide is virtually identical for practical purposes, and sodium chloride
also has similar properties. Ammonium chloride is an effective restrainer, but gives off a strong ammoniacal smell and may shift print color toward the red. Potassium iodide is a powerful
restrainer that gives blue-black tones, but requires longer fixing times. Two organic restrainers are occasionally utilized, benzotriazole and 6-nitrobenzimidazole nitrate, which are sometimes
referred to as antifoggants. Organic antifoggants shift print color slightly toward the blue. All restrainers tend to reduce emulsion speed and so are used sparingly. Mixing Photographic Solutions Distilled water is recommended for film developers, as even minute quantities of impurities can cause negative defects. Distilled water can be heated on the stove to the necessary mixing temperature, and small quantities can be kept refrigerated for adjusting solution temperatures downward. Tap water is generally sufficient for mixing paper developers. Some chemicals mix perfectly well at room temperature, but many require hot water to dissolve completely, so most developer formulas call for water at 125° Fahrenheit. Generally one begins with water at 75% of the liquid volume desired, so if one liter of solution is to be mixed, one begins with 750 milliliters of water. After the chemicals are added, the solution is topped up to the desired volume with cold water. Chemicals should be added to water slowly, one at a time, while stirring constantly. Each chemical must be thoroughly dissolved before the addition of the next. Tiny bubbles are formed as chemical reactions take place during the mixing process, and the liquid should be stirred until these bubbles dissipate and the solution clears before addition of the next chemical. This inevitably requires a great deal of stirring and no small amount of patience. Developer chemicals are usually mixed in the following order: (1) preservative The primary exception to the above rule is metol, which is always mixed first because it does not dissolve well in alkaline solutions. Most formularies recommend adding a pinch of sulfite before mixing the metol to retard oxidation. Always mix chemicals in the order given in the formula. Store developer solutions in brown glass or plastic bottles, with little or no air trapped in them. Several small bottles are preferable to one large one--the less air a developer is exposed to, the longer it will keep. Glass is better than plastic because it does not breathe. Safety
Pyrogallol, pyrocatechin, amidol, and paraphenylene diamine are highly toxic and readily absorbed through the skin--a few drops now and then will not be dangerous, but effects are often cumulative, so gloves and eye protection should be worn when working with solutions of these agents. Stock solutions of pyrogallol should be mixed under a chemical vent hood or out-of-doors to prevent the concentration of toxic fumes in the darkroom. Amidol should never be mixed into a solution much above 80 degrees Fahrenheit for the same reason. Concentrated acids and caustic alkalis should be handled with the greatest care. Though such chemicals are rarely used in the modern darkroom, they are not unknown--acid proof gloves are
recommended. Water should not be In general, gloves and eye-protection should be worn when handling caustic or toxic chemicals, and a NIOSH-approved face mask should be employed when mixing any chemical that might form dust. For further information on chemical safety, see Photographic Processing Hazards and Chemical and Other Safety Information. There are also some excellent books available, including OvereXposure and Health Hazards for Photographers. D-23 is an ideal developer for mixing because it is a simple two-ingredient formula that is relatively cheap and versatile--since it doesn't come in premixed form there is no other way to obtain it than by mixing your own. D-23 contains 7.5 grams of metol developing agent and 100 grams of sodium sulfite preservative. The sulfite provides sufficient alkalinity to activate and maintain the developing process, and in addition acts as a solvent. The solvent action is two-fold. First, sulfite partially dissolves exposed silver halide grains, allowing the developing agent to get at internal latent image specks where development must begin, allowing complete development of exposed halides and reducing speed loss. Secondly, when the sulfite dissolves exposed or unexposed silver halides it frees up metallic silver which is then plated back onto partially-developed grains in the emulsion in a process known as physical development--this tends to blur the rough outlines of the silver, giving a soft, semi-fine-grain effect. Metol by itself is a soft-working, low-fog agent, and with the low alkalinity of the sodium sulfite plus its anti-oxidant qualities, D-23 is almost guaranteed not to block up high values even when long development times are required. This is the developer Ansel Adams chose for his "Moonrise, Hernandez, New Mexico" negative. He was concerned that the moon would come out overdeveloped and not show any detail. Later he said if he had known how dark the foreground area was he would have given the whole negative another stop of exposure. Adams couldn't find his exposure meter in his hurry to make the shot before the sun set behind him. He later used a silver-based intensifier to enhance the foreground of this famous negative. But under normal circumstances, where correct exposure is given, D-23 renders excellent shadow detail. D-23 is sometimes used as a one-shot developer and then discarded. Kodak does however provide a replenisher formula, known as DK-25R, which allows the developer to be used for up to 26 rolls . One of the things that happens when the replenishment method is used is a buildup of silver in the developer solution. In the past, developers like D-23 were often replenished continuously, far beyond the recommended film capacity, and negatives were generally developed by inspection. When silver content builds up in D-23 its functionality is radically altered. Physical development takes place in direct proportion to the amount of silver developed out in the emulsion, causing an intensifying effect that is pronounced in the high values. Though unpredictable, this effect was sometimes exploited. Gallic acid was used by Fox Talbot as early as 1840 to sensitize as well as develop his paper negatives, but proved to be an extremely slow developing agent. By 1838 it had already been discovered that when gallic acid is heated to 200-250 degrees Fahrenheit it becomes more active. In early chemistry, substances were often heated to see what new properties they might acquire, and these heated substances were then prefixed with the Greek word pyro, meaning fire. So heated gallic acid was referred to (incorrectly) as pyrogallic acid. Actually, heating gallic acid liberates carbon dioxide, leaving 1-2-3 trihydroxybenzene or pyrogallol. Pyrogallol found use as early as 1851 in the collodion wet plate process, and continued to be used for the rest of the 19th century and well into the 20th in both wet and dry processes. Gordon Hutchings goes so far as to say that pyrogallol was the most popular developing agent of the 19th century, despite the many difficulties associated with its use. Though it was justly famous for its tonal gradation and rendering of fine detail, it was also known for its tendency to oxidize rapidly and cause mottling, streaks, and stains. With the discovery of hydroquinone in 1880, metol in 1891, and the subsequent invention of metol -hydroquinone formulas, pyrogallol began to fall into disfavor. While it was discovered that metol could be added to pyrogallol to increase film speed and contrast, inconsistent staining of the negative by oxidation byproducts continued to be a major problem; and, though non-staining formulas were developed, it was quickly discovered that many of the advantages of pyro over other developing agents resulted from the very stain that had been eliminated. Pyro continued to be used by a few fine art photographers and portraitists who appreciated its unique qualities, and by a few press photographers who required the speed of development delivered by certain pyro-metol formulas, but for popular use with miniature films (i.e., 35mm) pyro was almost completely supplanted by metol-hydroquinone. Gordon Hutchings appears to have done more research on pyro than anyone in recent history, and his The Book of Pyro is definitive. Hutchings has developed a formula that maximizes the stain, but allows it to take place in a controlled manner. His PMK formula (which stands for pyro-metol-kodalk), is now justly famous and continues to gain adherents among fine art photographers. The advantages offered by pyro are many, and far outweigh the disadvantages. The yellow-green color of pyro's stain adds effective density to the negative (which can only be accurately measured with a color densitometer), but unlike mere overdevelopment, which tends to increase graininess and mask fine detail, the stain serves to fill gaps between silver grains, thereby partially masking grain and providing a uniquely smooth tonal gradation. Pyro is unequalled in its ability to effectively render fog and mist. Due to the density added by stain, development time can be reduced to allow for translucent negative highlight values, thereby producing high-value detail that cannot be acheived with non-staining developers. Reduced development in turn restricts the possibility of contagious development and virtually eliminates fog. Pyro's tanning effect on gelatine helps to reduce grain migration and inhibits diffusion of bromide out of the emulsion. This gives the pyro enhanced adjacency effects and extremely high acutance--the detail it renders sometimes seems almost unreal. Because, with PMK, the staining action takes place within the emulsion rather than as a result of oxidation in the developer, the stain is directly proportional to the amount of silver reduced. The unwanted, random stain of older pyro formulas is eliminated. The staining and tanning of the emulsion also allow extended inspection times. Pyro formulas oxidize rapidly and must be mixed just prior to use, but PMK is a simple two-solution formula that keeps extremely well. Pyro is quite toxic--long term exposure can cause kidney disease and is probably carcinogenic. Recent research on Parkinson’s Disease has centered on toxins and other environmental factors. It is conceivable that Edward Weston's health problems in later life were the result of long-term exposure to pyro, amidol, and other photographic chemicals. There is a photograph of him by Ansel Adams that shows his fingernails black with amidol stains. But it is easy to over-react--gloves and eye-wear are adequate protection when handling pyro solutions. An occasional few drops of dilute solution on the skin can be flushed with water and are no cause for alarm. Long-term exposure is another matter, as the effects are often cumulative. Direct ingestion of even a gram of pure pyrogallol would certainly be fatal. A NIOSH-approved mask for hazardous dust should be worn when handling the dry chemical. Mix outdoors and do not breathe the fumes. For those who do not wish to handle pyrogallol, PMK is available pre-mixed from Photographer's Formulary and Bostick & Sullivan (in the U.S.), and Silverprint (in the U.K.). Pyrocatechin seems to be the preferred name for this agent, but catechol is sometimes more convenient as it prevents confusion with pyrogallol. Because of the tanning effect of catechol on emulsion gelatine, it has many of the properties of pyrogallol, including high acutance and low fog. Both are essentially surface developers: the hardened emulsion prevents development of halides below the surface, thereby reducing halation effects. Halation is caused by stray light bouncing around inside the gelatine layer and exposing crystals that would not otherwise receive exposure, but halation takes place almost exclusively in the depths of the emulsion so its effects are largely negated by surface developers. Catechol is a much underutilized agent, and formulas for it are rarely found. Its primary use has been as a compensating developer for subjects with extreme scales in the ten to fifteen stop range. Such extreme exposure value ranges are actually quite common in the Southwest. Ansel Adams gives an excellent two-solution catechol formula in his book The Negative. He states that it reduces emulsion speed considerably in the low values, so it is necessary to give twice the normal exposure. A more modern formula is Sandy King’s Pyrocat-HD, which was developed as a substitute for PMK that provides shorter printing times for alternative processes while still retaining the benefits of a staining developer. Apparently the color of the PMK stain inhibits ultraviolet light more than the brown stain of catechol developers. Ansco 130 and Adams' Ansco 130 Variant Ansco 130 is an old paper developer, combining glycin with metol and hydroquinone, specifically formulated to give warm tones. It gives a warmer tone on most papers than a straight metol -hydroquinone formula (such as Dektol or D-72), but is at its best with warm-tone chloride papers such as Agfa Portriga. In fact, I find that with many modern papers Ansco 130 gives remarkably neutral tones, particularly when fresh--it definitely gives warmer tones as it ages. Chloride papers are typically used today for portraiture because of their excellent rendering of middle-values, particularly skin tones. Undiluted Ansco 130 produces very deep blacks and startling high values--it is particularly useful where there is a need to boost contrast slightly, though it often proves too contrasty for normal prints. At its standard dilution of 1:1 it is an excellent all-purpose developer, producing a wonderful long scale on chloride papers. Glycin is a less expensive developing agent than it seems at first glance because it is very long -lasting in solution. Ansco 130 will keep for many months and can be used again and again. As it ages, the solution oxidizes somewhat and turns darker--itt is still perfectly functional as a developer, though it tends to produce less contrast and a warmer image. Prints developed in Ansco 130 should always be developed face up to prevent unwanted stains. The hydroquinone in the formula breaks down and ceases to produce its superadditive effect with the metol, but the metol and glycin continue to function long after the hydroquinone is exhausted. Ansel Adams probably got the idea for his variant from using Ansco 130 well beyond the effective life of the hydroquinone.
Glycin is moderately toxic, irritates the skin, and stains fingers and clothing. Gloves should be worn when working with glycin formulas. The pure chemical is a nearly white crystalline powder which darkens as it ages. In this author’s experience, when it becomes dark brown it is too oxidized to use as a film developer, but may still work as a paper developer, though with a somewhat reduced level of activity. However, Wynn White states the he uses dark brown glycin in his film developer with no apparent problems. The Photographer's Formulary is the only company which still produces glycin in the United States, and they make it in small enough batches that it is generally fresh when purchased. The mystique of amidol endures, despite the difficulties involved in its use. The chemical is quite poisonous, doesn't keep, stains fingers and clothing, and is the most expensive developing agent still in use. Adams never used it much, stating he felt he could get as good or better results with other formulas, though Edward and Brett Weston used it all their lives and a number of fine art photographers continue to maintain that it has certain unique but undefinable qualities that make it the most desireable of developing agents for papers. Michael Smith asserts that it is the best developer for Kodak Azo paper. Early in the 20th century (according to John S. Carroll) Amidol was prized for the blue-black tones it produced on pure bromide papers. Most modern papers are mixed chloro-bromide emulsions, each one differing slightly in composition, and only experimentation will reveal what sort of tones amidol may produce on each one. Typically, however, Amidol gives a rather greenish hue with chloride and chloro-bromide papers, particularly if restrained with potassium bromide. Selenium toning will transform this to a warm purple-brown. Agfa Brovira is said to be the only pure bromide paper still in production, and amidol formulas do indeed give cold blue-black hues with this paper. [Brovira has been removed from the market since this was written.] Interestingly enough, Steve Anchell states that “...amidol works best with only two types of paper. Either old-style, soft emulsion papers, or long-scale chloride papers, such as Kodak Azo and Bergger Art Contact.” He further states that amidol does not work well with modern papers because their emulsions are hardened for machine processing, but that Cachet Expo and Bergger Prestige papers are exceptions. (The Darkroom Cookbook, 2nd Edition, pp. 77-79.) In the early part of the 20th century, amidol was the developing agent of choice for making bromoil matrices. Amidol has also long been famous for the depth of its blacks. Modern tests indicate that there are several formulas that produce blacks that are equal to or greater in density than those produced by Amidol formulas, but true afficionados of amidol insist that subjective affect is more important than scientific proof when it comes to art. In truth, prints made in amidol give an impression of greater depth, and have remarkable tonal separation. Almost every manufacturer of chemicals and paper has produced an amidol formula at one time or another, and the vast majority of them give very similar results. One modern formula stands out from the rest--that of Samuel Fein. Mr. Fein chose to use benzotriazole as an anti-foggant rather than potassium bromide, and to add a significant quantity of citric acid. The citric acid prevents premature oxidation of the developer and eliminates unwanted stains, while the benzotriazole gives a much colder hue, even with chloro-bromide papers. This is definitely a developer worth experimenting with, though it is expensive. Another interesting formula is the Peckham Amidol, which contains pyrocatechin--it is very clean working and economical, compared to other amidol formulas. Amidol solutions do not keep and must be mixed just prior to use. However, everything but the developing agent may be pre-mixed. Amidol is a powerful developing agent that maintains full strength for lengthy printing sessions, and in combination with citric acid, amidol solutions will keep for several days. Hence, if you have a lot of prints to make, amidol is not as expensive as it may seem at first glance. Amidol in its pure form is a white, fluffy crystalline powder. As it ages it turns grey, then black, and becomes increasingly difficult to dissolve fully. Gloves should be worn when working with amidol solutions, and the developing agent should
never be added to a hot solution, as it gives off noxious fumes. It is wise to leave a half-inch to an inch margin around the print because tongs or gloves which are dipped in amidol developer and
exposed to air tend to stain the print. This article copyright 1990-2002 by Ed Buffaloe.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||
|
|
|
|



 Metol (N-Methyl-p-aminophenol sulfate) is a readily water-soluble, non-staining
agent which was introduced in 1891. With the addition of a small amount of bromide, metol is very clean working and has excellent keeping qualities. It is relatively
unaffected by low temperatures, but will not dissolve in alkaline solutions. In carbonate solutions it gives rapid development times which may be extended by
dilution. With sulfite alone, or mild alkalis such as borax or sodium metaborate, it produces a slow, fine grain developer for films. Metol by itself is very soft-working, but is
superadditive with hydroquinone, the combination of the two producing considerably more contrast than metol alone. Metol-hydroquinone is the most widely used combination for both film
and paper development. Metol is moderately toxic by ingestion, inhalation, or absorption through the skin. It may cause severe skin irritation and allergies in some users.
Metol (N-Methyl-p-aminophenol sulfate) is a readily water-soluble, non-staining
agent which was introduced in 1891. With the addition of a small amount of bromide, metol is very clean working and has excellent keeping qualities. It is relatively
unaffected by low temperatures, but will not dissolve in alkaline solutions. In carbonate solutions it gives rapid development times which may be extended by
dilution. With sulfite alone, or mild alkalis such as borax or sodium metaborate, it produces a slow, fine grain developer for films. Metol by itself is very soft-working, but is
superadditive with hydroquinone, the combination of the two producing considerably more contrast than metol alone. Metol-hydroquinone is the most widely used combination for both film
and paper development. Metol is moderately toxic by ingestion, inhalation, or absorption through the skin. It may cause severe skin irritation and allergies in some users.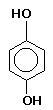 Hydroquinone (p-Dihydroxybenzene), discovered in 1880, is a clean-working, non-staining agent that is readily soluble in warm water (somewhat less so in cold), and is
extremely sensitive to the effects of low temperature and bromides. In highly alkaline solutions it produces a high speed, high contrast developer suitable for process work. In
combination with metol or phenidone, and carbonate, it produces a virtual universal developer which can be adjusted for almost any use. Hydroquinone is probably carcinogenic and
is highly toxic by ingestion. It is moderately toxic by inhalation and absorption through the skin..
Hydroquinone (p-Dihydroxybenzene), discovered in 1880, is a clean-working, non-staining agent that is readily soluble in warm water (somewhat less so in cold), and is
extremely sensitive to the effects of low temperature and bromides. In highly alkaline solutions it produces a high speed, high contrast developer suitable for process work. In
combination with metol or phenidone, and carbonate, it produces a virtual universal developer which can be adjusted for almost any use. Hydroquinone is probably carcinogenic and
is highly toxic by ingestion. It is moderately toxic by inhalation and absorption through the skin.. non-staining, highly active agent of the pyrazine series. Phenidone is
only moderately soluble in hot water, though it dissolves reasonably well in an alkaline solution. Due to its high activity in alkaline solutions it sometimes requires
considerable restrainer--often benzotriazole in addition to potassium bromide. In carbonate solution it produces a fast but soft working developer. With borax or sodium
metaborate it makes a fine grain developer. In combination with hydroquinone it becomes a normal contrast all purpose developer with excellent keeping properties. It
has taken the place of metol in many formulas because it is superadditive with hydroquineone, is much less likely to cause skin irritation, and only requires about 10% as much by weight.
Phenidone is moderately toxic by ingestion and inhalation, but only slightly toxic by absorption. It is generally considered to be the safest developing agent available other than ascorbic acid.
non-staining, highly active agent of the pyrazine series. Phenidone is
only moderately soluble in hot water, though it dissolves reasonably well in an alkaline solution. Due to its high activity in alkaline solutions it sometimes requires
considerable restrainer--often benzotriazole in addition to potassium bromide. In carbonate solution it produces a fast but soft working developer. With borax or sodium
metaborate it makes a fine grain developer. In combination with hydroquinone it becomes a normal contrast all purpose developer with excellent keeping properties. It
has taken the place of metol in many formulas because it is superadditive with hydroquineone, is much less likely to cause skin irritation, and only requires about 10% as much by weight.
Phenidone is moderately toxic by ingestion and inhalation, but only slightly toxic by absorption. It is generally considered to be the safest developing agent available other than ascorbic acid.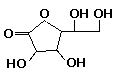 Ascorbic Acid (vitamin C) is a clean-working, non-staining agent, which is readily dissolved in water at room temperature. Ascorbic acid is typically used as a
replacement for hydroquinone, in combination with phenidone or the aminophenols, with which it is superadditive. It is also effective as a preservative and can be
used in formulas where it is desirable to reduce sulfite content. The oxidation byproducts of ascorbic acid are acidic, tending to inhibit further development in nearby areas; hence ascorbic
acid is has useful properties where adjacency effects or compensating development are desired.
Ascorbic Acid (vitamin C) is a clean-working, non-staining agent, which is readily dissolved in water at room temperature. Ascorbic acid is typically used as a
replacement for hydroquinone, in combination with phenidone or the aminophenols, with which it is superadditive. It is also effective as a preservative and can be
used in formulas where it is desirable to reduce sulfite content. The oxidation byproducts of ascorbic acid are acidic, tending to inhibit further development in nearby areas; hence ascorbic
acid is has useful properties where adjacency effects or compensating development are desired.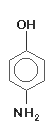 Paraminophenol, introduced in 1891, is much better known by the trade name Rodinal, the formula in which it is most often used. It is a fast-working, fog-free, stain-free agent that
dissolves easily in cold water, keeps extremely well and produces negatives of very high acutance. P-Aminophenol is much less sensitive than most other agents to temperature, and
does not cause fog at high temperatures. It is used primarily in highly concentrated pre-mixed solutions, and is seldom found in home darkrooms because pre-mixed Rodinal
remains cheap and readily available. It is highly toxic by ingestion and moderately toxic by inhalation or absorption. Prolonged contact may cause skin irritation.
Paraminophenol, introduced in 1891, is much better known by the trade name Rodinal, the formula in which it is most often used. It is a fast-working, fog-free, stain-free agent that
dissolves easily in cold water, keeps extremely well and produces negatives of very high acutance. P-Aminophenol is much less sensitive than most other agents to temperature, and
does not cause fog at high temperatures. It is used primarily in highly concentrated pre-mixed solutions, and is seldom found in home darkrooms because pre-mixed Rodinal
remains cheap and readily available. It is highly toxic by ingestion and moderately toxic by inhalation or absorption. Prolonged contact may cause skin irritation.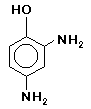 may be improved by the addition of boric, lactic or citric acid. Amidol was the paper developer of choice for Edward and Brett Weston, and is still cherished by many fine-art photographers. It
was famous fifty years ago for the blue-black tones it produced on bromide papers. Amidol possesses the unusual quality of being an active developing agent in a sulfite
-only solution, without the addition of carbonate, and remains active even in slightly acidic environments. It is also said to begin its development in the depth of the emulsion rather
than on the surface as do most agents. Amidol is highly toxic by inhalation or ingestion, and moderately toxic by absorption. Skin contact may cause severe irritation.
may be improved by the addition of boric, lactic or citric acid. Amidol was the paper developer of choice for Edward and Brett Weston, and is still cherished by many fine-art photographers. It
was famous fifty years ago for the blue-black tones it produced on bromide papers. Amidol possesses the unusual quality of being an active developing agent in a sulfite
-only solution, without the addition of carbonate, and remains active even in slightly acidic environments. It is also said to begin its development in the depth of the emulsion rather
than on the surface as do most agents. Amidol is highly toxic by inhalation or ingestion, and moderately toxic by absorption. Skin contact may cause severe irritation.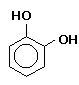 sodium hydroxide. It is considerably more susceptible to the influence of bromide in carbonate solutions. Pyrocatechin oxidizes very readily, particularly in the
absence of a sulfite preservative; the oxidation byproducts have a tanning effect on the gelatine of photographic emulsions, which tends to harden and stain them. This tanning
effect is proportional to the amount of silver reduced and is therefore greatest in the highlight areas which have received the most exposure. Local tanning and hardening tends to
inhibit further developing action in the area, making pyrocatechin an ideal compensating developer. The tanning effect also prevents development below the surface of the emulsion. Pyrocatechin is
highly toxic by ingestion, inhalation, or absorption through the skin. It is classified as a carcinogen. Skin contact may cause eczematous dermatitis. Pyrocatechin is combustible and gives off
irritating fumes when it burns.
sodium hydroxide. It is considerably more susceptible to the influence of bromide in carbonate solutions. Pyrocatechin oxidizes very readily, particularly in the
absence of a sulfite preservative; the oxidation byproducts have a tanning effect on the gelatine of photographic emulsions, which tends to harden and stain them. This tanning
effect is proportional to the amount of silver reduced and is therefore greatest in the highlight areas which have received the most exposure. Local tanning and hardening tends to
inhibit further developing action in the area, making pyrocatechin an ideal compensating developer. The tanning effect also prevents development below the surface of the emulsion. Pyrocatechin is
highly toxic by ingestion, inhalation, or absorption through the skin. It is classified as a carcinogen. Skin contact may cause eczematous dermatitis. Pyrocatechin is combustible and gives off
irritating fumes when it burns.  with the addition of metol or phenidone it produces normal contrast negatives. Pyro
oxidizes readily and the oxidation byproducts tan the gelatine of the emulsion, producing a distinct yellow-green stain in direct proportion to the amount of silver
halide reduced. This has the effect of somewhat masking image grain and improving detail in high values. The stain can be eliminated by the addition of sulfite in the ratio
of 4 or 5 to 1 of pyro. It is also possible to bleach out the silver and print by the stain alone. Once widely used, pyro formulas fell out of vogue due to the difficulty of controlling the
staining effect. However, the Wimberley WD2D formula and Gordon Hutchings' PMK (pyro-metol-kodalk) formula both give excellent control over the staining action and have brought pyro into
widespread use once again, particularly by fine art photographers. Pyrogallol is highly toxic by ingestion, inhalation, or absorption.
with the addition of metol or phenidone it produces normal contrast negatives. Pyro
oxidizes readily and the oxidation byproducts tan the gelatine of the emulsion, producing a distinct yellow-green stain in direct proportion to the amount of silver
halide reduced. This has the effect of somewhat masking image grain and improving detail in high values. The stain can be eliminated by the addition of sulfite in the ratio
of 4 or 5 to 1 of pyro. It is also possible to bleach out the silver and print by the stain alone. Once widely used, pyro formulas fell out of vogue due to the difficulty of controlling the
staining effect. However, the Wimberley WD2D formula and Gordon Hutchings' PMK (pyro-metol-kodalk) formula both give excellent control over the staining action and have brought pyro into
widespread use once again, particularly by fine art photographers. Pyrogallol is highly toxic by ingestion, inhalation, or absorption.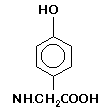 Glycin ( p-Hydroxyphenyl glycine), sometimes referred to as glycine, is a slow
-working, low-contrast, long-lasting agent that is quite sensitive to the addition of bromide as well as to low temperatures. Glycin causes no staining action when used
with film. It oxidizes very slowly (but old solutions can cause stains on papers). Glycin dissolves readily in alkaline or acetic solutions, but is virtually insoluble in
plain water. It is considered the most even developing agent for use with films, so is often used in stand developers such as Crawley’s FX-2, and is particularly suitable for continuous use in large
tanks. Glycin is known to produce warm tones on bromide papers, particularly in combination with hydroquinone. Glycin is moderately toxic by ingestion, inhalation, or absorption, and may cause skin irritation.
Glycin ( p-Hydroxyphenyl glycine), sometimes referred to as glycine, is a slow
-working, low-contrast, long-lasting agent that is quite sensitive to the addition of bromide as well as to low temperatures. Glycin causes no staining action when used
with film. It oxidizes very slowly (but old solutions can cause stains on papers). Glycin dissolves readily in alkaline or acetic solutions, but is virtually insoluble in
plain water. It is considered the most even developing agent for use with films, so is often used in stand developers such as Crawley’s FX-2, and is particularly suitable for continuous use in large
tanks. Glycin is known to produce warm tones on bromide papers, particularly in combination with hydroquinone. Glycin is moderately toxic by ingestion, inhalation, or absorption, and may cause skin irritation.
 1930’s. It is a slow-working staining developer, readily soluble in water, that has low
reduction potential and gives low contrast images in a sulfite sulution. With the addition of borax or carbonate it gives higher contrast but may generate dichroic fog. In a very alkaline
environment such as is provided by sodium hydroxide, paraphenylene diamine becomes a powerful, rapid developer. It has excellent keeping properties, but is poisonous, stains
fingers and utensils, and can cause skin allergies. Because it has a solvent action on silver, its primary application has been in fine-grain developers. It is almost always used in combination
with other agents that produce higher contrast. p-Phenylenediamine is highly toxic by ingestion or inhalation, and moderately toxic by absorption through the skin. It may cause severe allergies. It
gives off a toxic gas when combined with strong acids.
1930’s. It is a slow-working staining developer, readily soluble in water, that has low
reduction potential and gives low contrast images in a sulfite sulution. With the addition of borax or carbonate it gives higher contrast but may generate dichroic fog. In a very alkaline
environment such as is provided by sodium hydroxide, paraphenylene diamine becomes a powerful, rapid developer. It has excellent keeping properties, but is poisonous, stains
fingers and utensils, and can cause skin allergies. Because it has a solvent action on silver, its primary application has been in fine-grain developers. It is almost always used in combination
with other agents that produce higher contrast. p-Phenylenediamine is highly toxic by ingestion or inhalation, and moderately toxic by absorption through the skin. It may cause severe allergies. It
gives off a toxic gas when combined with strong acids.


