 |
|||
|
|
|
Novel Diapositive Images Harvey W. Yurow Ph.D.
In addition to permanganate and dichromate, a number of other bleach oxidizing agents, such as benzoquinone, cerate, and persulfate, have been reported for reversal procedures (Lumiere). In this paper, investigation was made of ceric sulfate, with regard to the phenomenon of re-reversal. As will be described below, the results obtained are novel. Background: Ceric ammonium sulfate (as an analytical oxidant) was first reported in 1861 by Lange (Kolthoff and Sandell). It is always used in strong acid solution, because ceric hydroxide is a weak base insoluble in water. An early application of cerium in photography was for a cerate silver bleach around 1900 in reports by the Lumiere brothers and Seyewetz. Primarily employed for reduction of dense negatives and prints, photographers utilized a solution of ceric sulfate in sulfuric acid. The mixture was extolled by Marsh and by Lea, who indicated that the concentrated solution (10%) keeps indefinitely, is usually diluted 1:10 for use, and that reduction increases contrast, which is much greater in the shadows than in the denser part of the image. In 1929, Seyewetz suggested the use of ceric sulfate for film reversal in hot climates, because of the resulting hardening of gelatin in the bleach (Clerc). In this connection, tetravalent cerium is a known gelatin hardening agent (Mees).
A detailed reference to ceric sulfate for film reversal was given by Glafkides, who wrote that ceric sulfate acted uniformly on the silver image, while permanganate and dichromate bleaches reacted mainly in the layer in the dense parts of the image. The author indicated that the bleach was made from 10g of ceric sulfate and 4cc of concentrated sulfuric acid per 1000cc of water, but had the disadvantage of being more costly than other bleaches. This formulation was derived from the commercial stock solution of the original Lumiere reducer, with its ceric sulfate concentration of 100g/l (Anon). As regards the relevant equation involved, Glafkides erroneously wrote that Ce3+ was reduced to Ce2+, but this should actually be Ce4+ to Ce3+. Haist made the same mistake in his text. The corrected equation is: 2H2Ce(SO4)3 +2Ag = Ag2SO4 +Ce2(SO4)3 + 2H2SO4 Additional references comment on the relatively slow reactivity of cerate when compared to permanganate and dichromate, although all three have similar high oxidation potentials (Salzberg,
Mees). This factor is suspected in being significant in the unusual results obtained with ceric sulfate as described below. By way of explanation, Paulenova indicated that in dilute sulfuric acid,
ceric ion is almost completely present as Ce(SO4)32- (the acid H2Ce(SO4)3 is orange), while colorless cerous ion is approximately half-complexed as Ce(SO4)+. Thus, the ceric ion is more
strongly bonded to sulfate ion than is the cerous ion. Consequently, Shibaoka and Hayashi described rapid reversal processing of microfilm with ceric sulfate bleach and trace amounts of aminothiol catalysts such as cysteine or thiourea, added to the first developer stop bath, because of the slow bleaching speed for silver of ceric sulfate alone. The authors also indicated that a cerium salt is a preferred bleaching agent for black and white films because it does not present pollution problems – i.e., is less harmful to animals and plants. Experimental: As indicated above, cerate compounds are considerably more expensive than are dichromate or permanganate salts. A recommended supplier is Antec Inc. in Louisville, Ky where anhydrous ceric sulfate (99%) is currently available at $65 for 100g including shipping. In the table below, as referenced from the literature, are five commonly encountered cerate solutions considered for the experiments:
In all of the above, except for the dot etching solution, sulfuric acid is added to water prior to addition of the ceric salt, in order to prevent the precipitation of basic ceric sulfates. The preparation of the dot etching solution is rather unconventional (NIIR Board)! To 67g of anhydrous ceric sulfate in a beaker is added 28cc of concentrated sulfuric acid with thorough mixing, followed by 30cc of water (spattering!). The mixture is heated and more water added in small amounts until the solid is completely dissolved, and the orange yellow solution becomes clear. The mixture is then made up with water to 1000cc. In initial and all subsequent experiments, the author used a modified Glafkides formulation from the above Table, with anhydrous ceric sulfate substituted for the tetrahydrate at 10g/l and sodium
bisulfate substituted for sulfuric acid at 50g/l (Bowler, Clerc). With actual usage, the solution In initial experiments with exposed Kodak T-Max 100 film, bleach removal of silver negative image was quite slow - in excess of 20 minutes reaction time, resulting from slow redox kinetics, as compared to 5 minutes or less for permanganate or for dichromate bleach. To speed up bleaching, following the work of Shibaoka and Hayashi, use of a first developer stop bath of acetic acid containing a small amount of catalyst such as thiourea (see below), considerably enhanced bleaching kinetics, but even at 10 minutes left a noticeably visible negative image, which did not interfere significantly with appearance of the positive. Unexpectedly, it was discovered that no second exposure was required to give satisfactory diapositives, in marked contrast to permanganate or to dichromate bleaching. An explanation for this behavior is that thiourea survived the cerate bleach and produced a modified Waterhouse Effect in the second developer, where formation of silver sulfide initiated development without second light exposure (see Discussion). Color renditions for various landscape objects of differing luminances with conventional Waterhouse (thiourea added to negative developer), and with the cerate-thiourea catalyst procedure with thiourea present in second development of a reversal process, are as follows:
Results from the above table, depending upon the degree of object luminance, are the combination of three separate processes, normal reversal, re-reversal, and the Waterhouse “thiourea blue” effect. This complex mechanism brings to mind Marshall McLuhan's comment on “startling changes resulting from new hybrids and crossings of media” (Yurow 2011). Of unusual visual appearance, high luminance objects in sunlight often posses a “spectral” quality, especially with structures having ornate facades. With regard to a visual comparison of the two equidensity procedures of interest , the Waterhouse technique frequently has an overall transmission of 5-7% and can be considered as dark (Milner) and more usually resembles a negative, while the cerate rendition in this study often results in a slide with approximately 20% transmission (brilliant) and is more like a positive. In connection with high luminance landscape objects, a number of photometric values have appeared in the literature (Adams, Encyclopedia of Colour Photography, James, Mack & Martin, Mees), and are given in the table below. Values reported in candles/ft. square have been converted to the metric value of millilamberts by a 3.38 multiplication factor. These subjects translate into lighter shades of blue as luminance values decrease, through off-white, and eventually into brown tones.
Most of the above in open shade (ca ¼ of the luminance in sunlight) will reproduce as off-white or tan to medium brown, while if in deep shade, will appear as dark brown. Some of the former may exhibit re-reversal, i.e. giving flat, featureless and grainy moderate luminance areas, which are quite noticeable, e,g., tree canopies, unpainted aged wood.
In Munsell color terms, shadows are rendered as ISCC-NBS color #47 – dark grayish red Brown, and highlights as ISCC-NBS #185 Blue (Manual). The actual reversal process used for T-Max 100, following a sunlit outdoor exposure with a Wratten #25 red filter of 1/125sec at f5.6, is as follows at 20-22 C.
Discussion: The elimination of any second exposure requirement with cerate bleach in the reversal process, which simplifies the procedure, is explained as follows. Assume, as also the case with dichromate bleaching, that the elimination of the sensitivity specks at the surface of the still unexposed silver halide crystals makes them almost insensitive to light (Kowaliski). Here, the Waterhouse effect comes into play, when thiourea with residual silver bromide forms silver sulfide in the second developer's basic solution to give silver sulfide specks which can act as developer initiators without the need of light (Yurow 2000). For this reaction to occur, it is required that thiourea or its silver bromide-thiourea complex survive in significant amounts attack by the oxidizing cerate bleach. This result seems plausible, because of the relatively slow oxidation kinetics of cerate ion as compared to those for permanganate and dichromate. In this connection, it was found that with too short a cerate bleach, i.e., 5 minutes, the significant amount of remaining negative image results in high fog and a dense diapositive. Conversely, too long a bleaching period , i.e., 15 minutes, oxidizes most of the thiourea complex, resulting in a brown diapositive lacking those blue highlights characteristic of the Waterhouse Effect (Yurow 2000). Consequently, 10 minutes bleaching time appears to be optimum. A batch of cerate bleach on second use, as compared to a freshly prepared sample, gives considerably deeper blue highlights, which may be related to the the significantly higher concentration of cerous ion present, which would slow oxidation kinetics. As regards outdoor first exposure, tonal results will vary considerably in the range 1/125 sec at f4, f5.6 and f8. Longer exposures will give a predominance of blue highlights over brown middle tones and shadows, while with shorter exposures, the opposite is true.
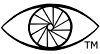
Middle tone re-reversals with cerate bleach and thiourea catalyst, are pictorially reminiscent of the tone separation process of Alfred Person. In brief, this latter technique involves preparation from an original negative of both specially processed intermediate positive and second negative, with subsequent printing in register of the two negatives. Here the results are images with striking middle tones that are flat and quite grainy, and are characterized by an unusual so-called “Z”shaped tone separation sensitometric curve (Romer, Kowaliski). With the current work, these flat areas can often be found in images of outer leaves of tree canopies, unpainted wood areas in open shade, etc.
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|