|
by Patrick Gainer
This article's title is my slightly humorous way of saying that I have concocted some developers for black-and-white film that contain ascorbic acid. You may recall that ascorbic acid is commonly called "Vitamin
C."
The Lesser Advantages
Being more than 65 years old has some compensations, even some joys. For example, I learned that prune juice bottles make fine wide-mouth containers for developer. Every once in a while, just when I think I know all
there is to know, I learn something that I didn't know before. As often as not, what I learn is that something I thought to be true is not. When it is something that a lot of other people also thought to be
true, then I think I have made a Great Discovery. Others make these Great Discoveries also, upon occasion. For example, once upon a time everyone knew that the tomato was deadly poison, and that one could
demagnetize a permanent magnet by rubbing garlic on it. I didn't discover the fallacies in those beliefs, but whoever did made some Great Discoveries. Now we can have pizza with tomato sauce, and we don't
have to worry about getting garlic on our refrigerator magnets.
An even greater advantage of being over 65, is that people think it is normal when you wander off the subject.
Those Greater Advantages
For manv years I have mixed developers from scratch, both for practical reasons and creative ones. My
formulas were usually other people's formulas, although I did some experimenting. I read what I could find,
both on that particular formula and on the theory of developers. Most of my experiments were based on the
usual rules: a developer needs, beside the "developing agent," a preservative, an accelerator, and sometimes a
restrainer. Developing agents most readily available were metol, hydroquinone, and Phenidone. The
preservative was a sulfite, usually of sodium, which sometimes served also as accelerator. If more alkalinity was needed, then borax, sodium metaborate, sodium carbonate, or even lye were used. Some of my
experiments were described in the article "Kitchen Tested Soups" published in the April 1973 issue of
Petersen's Photograpbic. The principal purpose of that article was to show that volumetric measurements of
solid photographic chemicals were quite satisfactory for most purposes. Upon reading that article, probably
believing it to be an April Fool's joke, a friend went out and spent much money on a balance for weighing his chemicals to the milligram.
I retired after 30 years of employment by NACA-NASA as an Aeronautical Research Scientist and am now living in wild and wonderful West Virginia, 50 miles from the nearest retail source of photographic supplies. It
takes from 7 to 14 days for shipments to arrive by UPS. For others in my predicament, it might be reassuring to know that a few grams of Phenidone are a lifetime supply, and that the other ingredients of a very good
developer for black-and-white film and paper can be obtained at a health food store and a supermarket.
Anyone who prepares developers from "scratch" knows that sodium sulfite and sodium carbonate are the
most expensive ingredients. In a gallon of D-76, for example, the metol may cost about 65 cents, but the sulfate can cost as much as $4.50. Not only that, but carbonate and sulfite are the chemicals you run out of
most often.
For many years, I used the washing soda form of sodium carbonate because it was cheap and readily
available. The last I got was so heavily perfumed I couldn't stand to have it in my print developer. In any case, none of the local stores have stocked it lately.
What About the Sulfite?
I found several ways around my odoriferous carbonate problem, but what could I do about the sulfite? When my sulfate began to run low, and I noticed that my wife hid purchased some ascorbic acid crystals from the
local health food store, I remembered that ascorbic acid is used to keep canned and frozen fruit from oxidation-related discoloration. Also, I had read many years ago something about 1-ascorbic acid forming a
superadditive pair with metol. At the time, 1 thought that 1-ascorbic acid was not the Vitamin C form. Later I learned that onlv 1-ascorbic acid has antiscorbutic properties. So, I decided to experiment.
What I learned, I wish I had known 25 years ago. After some considerable application of the scientific method--trial-and-error--I arrived at several sulfite-free developers for black-and-white materials, some with
metol and others with Phenidone.
If you do not have a balance, you can still use these developers because I will give quantities in volumetric as
well as weight units. If you are interested in purchasing a balance, I can recommend one made by Lyman for
weighing gunpowder and bullets. It is available in two forms at sporting goods stores--one calibrated in grains and the other in grams. It weighs accurately up to 500 grains and should cost about $35.
The Final Side-track
Before I describe my experiments, I should tell you of my alternate sources of sodium carbonate. First, any
department or hardware store that sells swimming pool supplies will probably sell "PH PLUS," a trade name of the Olin Company. The label says it is 98% sodium carbonate, and it seems to be anhydrous because
intense heating caused no loss of weight. It works and it costs 1/5 of what you get from other sources. I have no idea what the other 2% is except that it is claimed to be inert.
A second substitute is as follows. Any brand of baking soda is very pure sodiwn bicarbonate. Red Devil
brand of lye is very pure sodium hydroxide. If you mix one weight unit of lye with 2.1 weight units of baking soda in water, you will make a solution of very pure sodium carbonate. The chemical reaction is shown
below:
NAOH + NaHCO3 --> 4Na2CO3 + H20
The resulting solution is exactly the same as if vou had used 3.1 weight units of monohydrated sodium
carbonate. Lye is best handled in solution because of its propensity for picking up water and C02 from the air while you try to weigh it. The contents of one full 12-ounce (340 gram) can of lye, dissolved in water to
make 115 fluid ounces (3.4 liters), make a 10% solution which you can further dilute to suit your fancy. Start with very cold water, use a vessel that can stand some heat, and add the lye slowly to the water.
You can make a convenient 20% solution of sodium carbonate monohydrate by combining 64.5 g of lye (645ml of 10% solution) and 135.5 g of baking soda in water to make one liter. If your tap water is as hard
as my well water, use distilled water or rain water.
A third method of getting sodium carbonate locally, wherever you may be, is to heat baking soda. The
temperature required is quite high. I used a stainless steel saucepan on the stove top. When the baking soda
is hot enough, you will see little geysers of steam and carbon dioxide which are fun to watch, but which spray powder around. I convinced my wife that the white coating on the stove was an excellent degreasing agent,
but I still had to clean it up. Anyway, it is not good to inhale the powder, so put a lid on the pot. When you
agitate the contents with a swirling motion, it will feel as if there were liquid in the pan until all the gases have been given off. A pound of baking soda yields 10.6 ounces of anhydrous sodium carbonate, which is
equivalent to 12.4 ounces of monohydrated sodium carbonate. One tablespoon of this anhydrous carbonate weighs about 8.8 g.
One more thing before I proceed. Developers requiring Phenidone have such small amounts that it is convenient to make a solution of Phenidone in alcohol. You may use either denatured alcohol from the
hardware store or 91% isopropyl rubbing alcohol from the drugstore. Don't use the 70% stuff. Dissolve 1/4
teaspoon (0.65 g or 10 grains) of Phenidone in alcohol to make 80 ml. The Phenidone keeps quite well this way, which is a good thing as the 80 ml will make 8 gallons of film developer.
The Evolution of My Film Developers
I don't remember what my first trial was. I do remember that I learned very quickly that anything like the
usual concentrations of developing agents would be much too vigorous for practical use. I settled on 5g of anhydrous sodium carbonate, 2 g of ascorbic acid, and either 0.2 g of metol or 2.5 ml of Phenidone-alcohol
solution to make a quart of developer.
Either of these formulas is about as active as full-strength D-76, For example, I would give Tri-X 8 minutes at
68°F for normal scenes to be printed with a diffusion enlarger. Both formulas worked quite well on all the films I could get my hands on, including Ilford HP5-Plus and Delta 400; Kodak Tri-X, Plus-X, TMax 100, T
-Max 400, and T-Max 3200; and Arista Pro 400 and 125. The sharpness is excellent and the grain is surprisingly fine.
What is the chance that I got a special batch of ascorbic acid crystals? I tried other forms of Vitamin C, in
capsules and in tablets. What I learned is that tablets are hard as heck to dissolve, and I had to crush them to
do any good. Furthermore, there was still some insoluble flotsam and jetsam that you might want to filter out,
but the solution worked. Capsules would be convenient, because they contain measured amounts and they can be taken apart to get out the acid, but even though it is powdered, there was still some residue to be
filtered. Ascorbic acid crystals obtained from General Nutrition Centers (a national chain) will have 3%
obtained from rose hips, have a slightly pink color, and may leave some residue, but work as well. The purest
I have found is distributed by NOW Foods, (Glendale Heights, IL 60139). It is also the least expensive in mv area at $9.95 for a half pound.
Can the Ascorbic Acid be Replaced?
What is going on here? Is the ascorbic acid merely a preservative? Could it be replaced by sodium sulfite?
No, I tried. Given the same development time, the same concentrations of metol or Phenidone, and the same amount of carbonate, but substituting 2g of sulfite for the ascorbic acid, produced such a faint image as to be
almost invisible.
A little study of what might be the products of reactions in the developer solution, excluding the development
itself, turns up some interesting facts. While it would seem that the base in these experiments is sodium
carbonate, that is not exactlv the case. If there is more sodium carbonate than is required to convert the ascorbic acid to sodium ascorbate, the following equivalence holds:
xNa,C03 + C6H806 <--> C6H7NaO6 + NaHCO3 + (x-1) Na2CO3
(Where C6H806 is ascorbic acid, C6H7NaO6 is sodium ascorbate, and x is an undefined variable.)
The arrow goes both ways because there is no effervescence or precipitation. On the other hand, if there is an excess of ascorbic acid then the following holds true:
NA2CO3 + 2C6H806 --> 2C6H-,NaO6 + H20 + C02
In this case you get a lot of effervescence that continues as you add carbonate until all the acid is converted to
sodium ascorbate or all the carbonate has been added. It makes a difference, then, which you dissolve first.
In the first case, the result is equivalent to sodium ascorbate added to a buffer solution of sodium carbonate
-sodium bicarbonate and the pH is less than in the second case. If you base all quantities on 2 g of ascorbic acid, then 1.204 g of monohydrated sodium carbonate plus 2 g of ascorbic acid are exactly equivalent to 2
.239 g of sodium ascorbate plus 0.954 g of sodium bicarbonate, provided that you dissolve the carbonate first. The recipe that I arrived at by trial-and-error has about 3.8 g excess carbonate. Together with the
bicarbonate, it forms a pH buffer. Remember that 1 unit of sodium hydroxide plus 2.1 units of sodium bicarbonate are equivalent to 3.1 units of sodium carbonate monohydrate (or 2.65 units of anhydrous sodium
carbonate). The 3.8 g excess carbonate are equivalent to 3.Ol g of bicarbonate + 1.43 g of sodium hydroxide. With that in mind, I did a little research and found in the Handbook of Chemistry and Physics
tables for two buffer pairs that seemed appropriate: sodium bicarbonate-sodium hydroxide and borax-sodium hydroxide.
If the carbonate is dissolved first, the excess sodium carbonate in my original recipe along with the sodium bicarbonate formed in the conversion of ascorbic acid to sodium ascorbate are equivalent to 3.96g of
bicarbonate plus 1.43 g of hydroxide. This ratio corresponds to the ratio in a standard buffer solution of pH
approximately 10.8, but the concentrations are somewhat greater than standard. If the acid is dissolved first,
the bicarbonate is down to 3-01, and that ratio corresponds to pH 11.5 or greater. Sodium ascorbate does not contribute much to pH as even a 10% solution has pH between 7.4 and 7.7.
Out of curiosity, I decided to see what would happen if I used a standard buffer solution of pH=10.8 (as
nearly as I could make it) the base for my developer. According to my calculations, the developer would then have 2.81 g of bicarbonate, 1.1 g of hydroxide, and 2.25 g of sodium ascorbate per quart in addition to the
developing agent. If I use 2 g of ascorbic acid in place of the ascorbate I require 0.454 g more of the
hydroxide for a total of 1.55. This formula works as well as my original. Dissolve the bicarbonate and the lye before the ascorbic acid.
The pH value of 10.8 is within the range of a standard borax-sodium hydroxide buffer solution. 6.1 g of borax (20 Mule Team is just fine) plus either 1.7 g of hydroxide and 2 g of ascorbic acid or 1.25 g of
hydroxide and 2.25 g of sodium ascorbate, plus the developing agent make a quart of developer that is as active as the carbonate version. It has the advantage that it does not produce gas bubbles in acid stop bath.
Pure sodium ascorbate as an alternate form of Vitamin C for those who cannot tolerate the acidity of ascorbic acid is obtainable from NOW Foods. I have tested it and found that the aforementioned equivalences are
true.
Developers for Paper
Everyone knows that hydroquinone developers are slow unless the pH is so high you can't put your finger in
the solution. However, without sulfite hydroquinone is quite active even at moderate alkalinity, at least for a
little while. It seems that the sulfite, while preserving, also inhibits. Can we keep this activity and prolong it by using ascorbic acid in place of sulfite?
Mix 1 tsp (about 3 g) hydroquinone, 1/2 teaspoon ascorbic acid crystals and 1 tablespoon of sodium
carbonate (about 15 g) in a quart of water. Use it as a print developer. Be prepared for strange results.
Development takes a while to start, then accelerates. The result looks like some kind of solarization. I
believe what has happened is called "infectious development," a characteristic of some process developers,
but it has been so long since I read about it, I can't be sure. If so, it is one kind of infection that Vitamin C
can't cure. But add 2 ml of Phenidone-alcohol solution and try again. Now, you have a fairly decent print
developer. Do not mix any more than you can use in a couple of hours if you decide that it is worth using at all.
Actually, the hydroquinone is not needed. You can get all the density range your paper can give with metol or
Phenidone, ascorbic acid, sodium carbonate, and a little bromide. Use 1 tsp (4 g) of ascorbic acid, 1 tablespoon (15 g) of PH-Plus, and either 1/8 tsp of metol or 5 ml of the Phenidone solution to make a quart
of developer. Add bromide to suit. I use 5 drops of saturate solution.
The recipe for paper is almost a double-strength version of my film developer. In fact, if you make a
developer stock solution, just like the film developer but four times more concentrated, vou can dilute it 1+3 for film and 1+1 for paper. Even the borax formula.
Conclusions
 My recipes are summarized in the accompanying
Table I and Table II. You may draw your own conclusions, but I am quite convinced that ordinary ascorbic acid, obtainable as Vitamin C, has valuable photographic uses. It makes possible sulfite-free
developers with very low concentrations of the customary developing agents. It is certainly economical enough, and the major constituents are no strain on the environment. My recipes are summarized in the accompanying
Table I and Table II. You may draw your own conclusions, but I am quite convinced that ordinary ascorbic acid, obtainable as Vitamin C, has valuable photographic uses. It makes possible sulfite-free
developers with very low concentrations of the customary developing agents. It is certainly economical enough, and the major constituents are no strain on the environment.
I have included several photographs, the negatives for which were developed in one or another of my formulas. Some of the prints were made on ORWO 111, grade 2, sold by Freestyle as "Germany's Finest
." Others were made on Seagull Select variable contrast fiber base paper.
I have done some, but not much, sensitometric analysis. My equipment for the purpose is verv
limited. I used a cardboard box painted flat black inside with a hole in one side. This hole is about as near a black hole as you may see on earth. I cut a
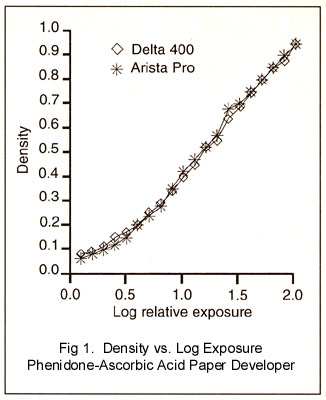
Kodak gray scale in half and mounted one half on either side of the black hole. Photographic negatives of the gray scale were projected by my C-760 dichroic enlarger and the projection densities were read using a
homemade (and designed) photometer calibrated in logarithmic units. You can see the results in Figure 1. The
densities in the plots are those the paper would see if I had made prints. They include the effects of flare in the camera lens and body (Canon AE-1, 85mm Canon FD lens), and other experimental artifacts.
|
|




 My recipes are summarized in the accompanying
Table I and Table II. You may draw your own conclusions, but I am quite convinced that ordinary ascorbic acid, obtainable as Vitamin C, has valuable photographic uses. It makes possible sulfite-free
developers with very low concentrations of the customary developing agents. It is certainly economical enough, and the major constituents are no strain on the environment.
My recipes are summarized in the accompanying
Table I and Table II. You may draw your own conclusions, but I am quite convinced that ordinary ascorbic acid, obtainable as Vitamin C, has valuable photographic uses. It makes possible sulfite-free
developers with very low concentrations of the customary developing agents. It is certainly economical enough, and the major constituents are no strain on the environment.